Israel to dose last BriLife Phase II trial participant on Sunday
The Phase II trial had been approved and kicked off in December 2020, around the time the first vaccines arrived in Israel
By MAAYAN JAFFE-HOFFMAN , Jerusalem Post, SEPTEMBER 18, 2021
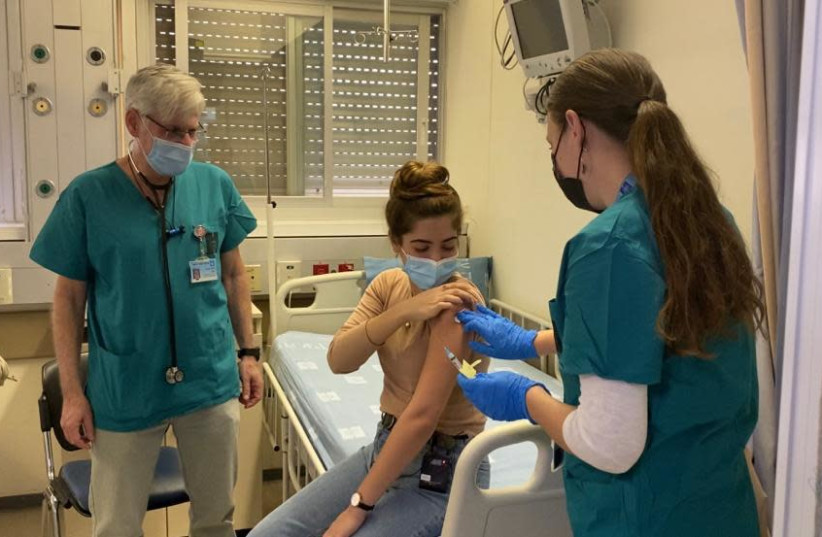
Hadassah-University Medical Center's Prof. Yossi
Karko (left) and Hannah Drori, chief of the hospital’s clinical research
center, administer Brilife vaccine to a volunteer (photo credit: HADASSAH)
The last volunteer of Israel’s BriLife vaccine Phase II clinical
trial will be dosed on Sunday, according to Eytan Ben-Ami, head of early
phase clinical trials at Sheba Medical Center.
The
data from the trial of more than 700 people should be analyzed in
October and then the research team will be able to make a decision about
whether or not to continue to Phase III.
The
Phase II trial was approved and kicked off in December 2020, around the
time that the first vaccines arrived in Israel. Ben-Ami said that the
volunteers would be monitored for a year.
The BriLife vaccine was developed by the Israel Institute for
Biological Research (IIBR) in Ness Ziona, which is under the auspices of
the Defense Ministry. The ministry in July inked a deal with US-based
pharmaceutical company NRx to help fast-track the vaccine.
BriLife
is currently undergoing a Phase IIb trial in Georgia that is being
overseen by NRx, which now has exclusive worldwide development,
manufacturing and marketing rights.
In
Israel, four doses of the BriLife vaccine were tested as part of the
Phase II trial – low, medium, high and a “top dose,” Prof. Yossi Caraco,
head of the Clinical Pharmacology Unit at Hadassah-University Medical
Center, explained. The highest dose seemed to be the most effective, he
said, and the results seem “very promising” – although no final
evaluation has been made.

A WOMAN receives a third dose of the COVID-19 vaccine, at Meuhedet
vaccination center in Jerusalem, last week. (credit: YONATAN
SINDEL/FLASH90)
BriLife
is a vector-based vaccine. The vaccine takes the vesicular stomatitis
virus (VSV) and genetically engineers it so that it will express the
spike protein of the novel coronavirus on its envelope.
Once injected, it does not cause a disease by itself. VSV does not
infect humans; instead, the body recognizes the spike protein that is
expressed on the envelope and begins to develop an immunological
response. The vaccine will initially be delivered by traditional
injection.
“The BriLife vaccine differs from other COVID-19 vaccines
by presenting the entire COVID-19 spike protein to the body’s immune
system,” explained NRx in a release. “It also differs from other
COVID-19 vaccine approaches in that it is a self-propagating, live-virus
vaccine in which the spike protein of the vaccine appears to evolve in a
manner consistent with the evolution of the SARS-CoV-2 virus in nature.
“Thus,
while variants may arise that support manual enrichment of the vaccine
against those specific variants, the vaccine itself may continue to
evolve in a manner that provides ongoing protection against variants,”
the release said.
Rx chairman Prof. Jonathan Javitt told The Jerusalem Post in August that the goal is to launch a Phase III trial by October. That trial will be across multiple countries, including Georgia and Ukraine, and will include up to 30,000 people.
This page was posted by Sputnik One of the Sputniks Orbit blog
Please Recommend this page and follow us


No comments:
Post a Comment