$15 treatment gets COVID patients off ventilators in under a week - study
Fenofibrate could dramatically shorten the treatment time for severe COVID patients.
By MAAYAN JAFFE-HOFFMAN , Jerusalem Post, AUGUST 23, 2021
Hebrew University Professor Yaakov Nahmias, (photo credit: DANIEL HANOCH)
Fourteen out of 15 severe COVID-19 patients who were treated in an investigator-initiated interventional open-label clinical study
of the drug TriCor (fenofibrate) didn’t require oxygen support within a
week of treatment and were released from the hospital, according to the
results of a new Hebrew University study.
Fenofibrate is an FDA-approved oral medication. The results were on Research Square and are currently under peer review.
Specifically,
the team that was led by HU’s Prof. Yaakov Nahmias carried out the
study at Israel’s Barzilai Medical Center in coordination with the
hospital’s head of the Infectious Disease Unit, Prof. Shlomo Maayan, and
with support from Abbott Laboratories
The 15 treated patients all had pneumonia and required oxygen
support. They were also older with multiple comorbidities, ranging from
diabetes and obesity to high blood pressure.
In addition to standard of care, the patients were given 145 mg/day of fenofibrate for 10 days.
“The
results were dramatic,” Nahmias told The Jerusalem Post. “Progressive
inflammation markers, that are the hallmark of deteriorative COVID-19,
dropped within 48 hours of treatment. Moreover, 14 of the 15 severe
patients didn’t require oxygen support within a week of treatment.”
The 15th patient was off oxygen within 10 days.
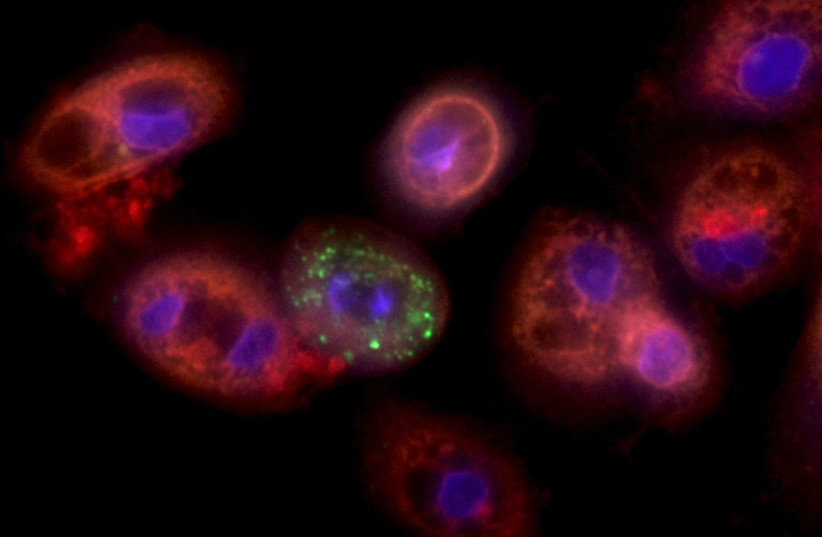 Lung cells infected with coronavirus (credit: YAAKOV NAHMIAS)
Lung cells infected with coronavirus (credit: YAAKOV NAHMIAS)He
said that when looking at the data on other similar severe patients, on
average, less than 30% of them are removed from oxygen support in a
week. In other words, fenofibrate could dramatically shorten the
treatment time for severe COVID patients.
“We
know these kinds of patients deteriorate really fast, develop a
cytokine storm in five to seven days and that it can take weeks to treat
them and for them to get better,” Nahmias said. “We gave these patients
fenofibrate and the study shows inflammation dropped incredibly fast.
They did not seem to develop a cytokine storm at all.”
Fenofibrate effective in treatment of COVID-19 | Prof. Yaakov Nahmias | Hebrew University
In general, patients that do not require oxygen can be treated at
home. Additionally, despite the high number of COVID deaths in Israel
and abroad, the majority of severely sick patients survive.
“If you look over a 28-day period, I would have expected all of them to survive with or without the drug,”
Nahmias explained. “The question is how fast we can get them home or
how quickly we can get a severe patient to a mild condition.”
All
of the patients completed a 10-day home treatment after discharge and,
according to Maayan, “no drug-related adverse events” were reported.
Fenofibrate
was approved by the FDA back in 1975 for long-term use and is
considered safe. Moreover, it is an inexpensive pill, Nahmias said. It
costs less than $1.50 a day, meaning that the entire treatment per
patient was around $15.
Nahmias
has been studying the use of fenofibrate for treating COVID-19 almost
since the start of the pandemic. He first ran a pre-clinical trial and
then a multi-center retrospective study, both of which supported the
effectiveness of the drug.
“Viruses
are parasites,” Nahmias explained. “They cannot replicate by
themselves. They have to get inside a human cell and hijack their
machinery to replicate.”
Working
with collaborators in the United States, Nahmias demonstrated that the
coronavirus prevents the burning of fat in lung cells, resulting in
large amounts of fat accumulating inside lung cells – a condition the
virus needs to reproduce. Fenofibrate, he hoped, would reverse that
effect, and eliminate virus replication.
“By
understanding how the SARS-CoV-2 controls our metabolism, we can
wrestle back control from the virus and deprive it of the very resources
it needs to survive,” Nahmias told the Post, noting that it also may
help explain why patients with high blood sugar and cholesterol levels
are often at a particularly high risk to develop COVID-19.
Now,
he is involved with a series of Phase III studies that are being
carried out in South America, the United States and Israel. Those
studies are placebo-controlled and double-blind.
Nahmias
said his team had been struggling to get patients enrolled in the study
before the onset of the Delta variant, but efforts are now more rapidly
progressing. He hopes that results could be available as early as
within the next two months.
In the meantime, the drug is available, and physicians can decide to give treatment with it based on available data.
“There
are no silver bullets,” he said, “but fenofibrate is far safer than
other drugs proposed to date, and its mechanism of action makes it less
likely to be variant-specific.”
This page was posted by Sputnik One of the Sputniks Orbit blog
Please Recommend this page and follow us


No comments:
Post a Comment